Tiny things matter—for instance, one amino acid can completely alter the architecture of the cell. Researchers at the Universities of Göttingen and Warwick investigated the structure and mechanics of the main component of the cell’s cytoskeleton: a protein known as actin. Actin is found in all living cells, with a range of important functions—from muscle contraction to cell signaling and shape.
This protein comes in two varieties termed “isoforms,” known as gamma-actin and beta-actin. The difference between the two proteins is minuscule; only a few amino acids at just one part of the molecule vary. Yet this small change has a big impact on the cell. In nature, normally, only mixtures of the two isoforms are found. In their study, the researchers separated out the two isoforms and analyzed them individually. The results were published in the journal Nature Communications.
The researchers studied the behavior of networks of filaments, particularly focusing on the unique properties of the individual isoforms. They employed specialized techniques allowing them to assess the mechanics and dynamics of research models of cytoskeletal networks, drawing on expertise in biophysics at Göttingen and bioengineering at Warwick.
The results indicate that gamma-actin prefers to form rigid networks near the cell’s apex, while beta-actin preferentially forms parallel bundles with a distinct organizational pattern. This difference is likely to be due to the stronger interaction of gamma-actin with specific types of positively charged ions, rendering its networks stiffer than those formed by beta-actin.
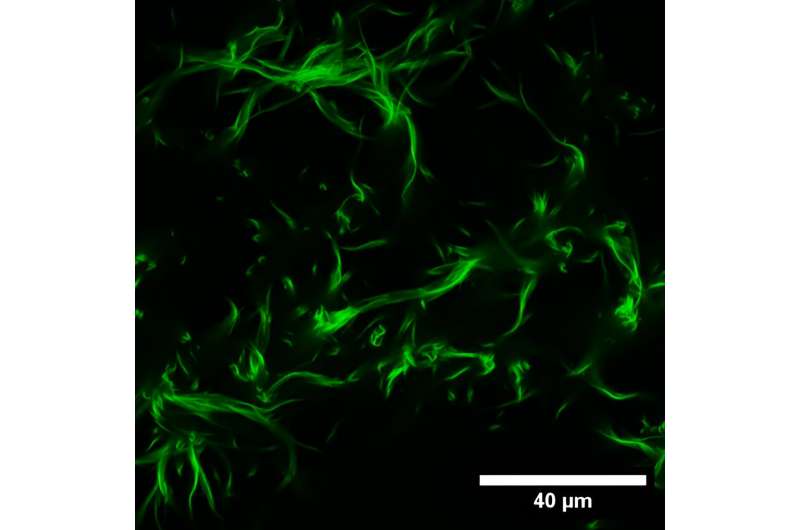
“Our findings are compelling because they open up new avenues for understanding the intricate dynamics of protein networks within cells,” explains Professor Andreas Janshoff, Institute for Physical Chemistry, University of Göttingen.
The research advances scientists’ understanding of fundamental cellular processes by shedding light on specific biological functions of actin, and this will have particular relevance for processes involving cellular mechanics such as growth, division, and maturation of cells in tissue.
“The implications of these discoveries extend to the broader field of cellular biology, offering insights that could impact many areas of research and applications, for instance, in developmental biology,” adds Janshoff.
More information:
Peter Nietmann et al, Cytosolic actin isoforms form networks with different rheological properties that indicate specific biological function, Nature Communications (2023). DOI: 10.1038/s41467-023-43653-w
Citation:
Big impacts from small changes: Research reveals how filament interactions affect cellular networks (2023, December 22)
retrieved 23 December 2023
from https://phys.org/news/2023-12-big-impacts-small-reveals-filament.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

